Scientists have been looking for medication for vascular malformations (blood vessel diseases) for a long time. The medicine sirolimus seems to have a beneficial effect. This was shown in studies with severe vascular anomalies. A study is currently underway in which children and adult patients with complex vascular malformations that respond poorly to the standard treatment are treated with sirolimus.
Prof. Dr. Laurence Boon and Prof. Dr. Miikka Vikkula (one of our advisors) are also involved in this development. They recently (15 June 2020) published a review article ‘New and Emerging Targeted Therapies for Vascular Malformations’ in the American Journal of Clinical Dermatology. In this article they give an overview of what is known so far about vascular malformations and the effect of the treatment of sirolimus and other specific drugs. Under the heading ‘New and emerging targeted therapies for vascular malformations’ (scroll down on this page) a concise summary of this article is given.
Within Facebook a separate private group is available ‘Sirolimus For Complex Vascular Anomalies’:
For which diseases?
The initial clinical use of sirolimus involved immunosuppression to prevent kidney transplant rejection and has extensively been studied in this context. It received US Food and Drug Administration and European Medicines Agency approval in 1999 and 2001, respectively, for this indication. Sirolimus belongs to the group of ‘Mammalian target of rapamycin (mTOR) inhibitors’. These inhibitors have further orphan indications in a large range of diseases such as soft-tissue and bone sarcoma, lymphoma, neuroen-docrine tumors, and tuberous sclerosis. Currently (June 2020) Sirolimus has not been approved for treatment of any vascular malformation. It has been used in a compassionate use setting for patients with complex life-threatening vascular anomalies. A phase III clinical trial (VASE) is currently underway in Europe.
How Sirolimus works
Sirolimus intervenes with an important signal route in our body. It is an inhibitor of the so-called mTOR signalling pathway. This pathway is important in all kinds of cellular processes such as cell growth, division and survival. The following proteins, among others, are found in this route: TIE2-> PI3K- (PTEN) -> AKT-> . Mutations in TIE2, PI3K, PTEn or AKT can (over)activate the route.
Scientific article in layman’s language
New and emerging targeted therapies for vascular malformations
Over the last twenty years a great deal of knowledge has been accumulated about the genetics and molecular mechanisms behind the development of vascular malformations. Until now, vascular malformations have mainly been treated by surgical procedures and embolisation (sealing of a vessel). Due to the increased knowledge about the underlying mechanisms, attempts are now being made to intervene on these mechanisms with specific medication. Especially sirolimus, also known as rapamycin, is the most studied compound and seems to have a beneficial effect. Until now, it has mainly been used in severe vascular anomalies.
Vascular anomalies are now mostly considered to be caused by abnormal signaling within vascular endothelial cells [The endothelium is a covering single cell layer of contiguous cells that covers the inside of the heart, blood vessels and lymphatic vessels in vertebrates].
Signal transfer enables that all kinds of commands can be transmitted within the cell, such as starting cell division, protein synthesis, movement, dying (or not). Signal transmission is provided by pathways/routes of mainly proteins. These proteins transmit the signal to each other. If a protein does not work (well) or works too well because it is abnormal, this has consequences for the signal transmission. The signal transfer stops, is too slow or too fast. As a result, for example, a too low or too high degree of cell division may occur.
The PI3/AKT/mTOR and RAS/MAPK/ERK pathways
The anomalous signal transmission is due to mutations in genes that activate the signal transmission. Most vascular malformations are caused by mutations in genes that cause activation of two important intracellular signaling pathways. They are the so-called PI3K/AKTmTOR pathway and the RAS/MAPL/ERK pathway. Both pathways are involved in many fundamental cellular processes such as proliferation, differentiation, motility, stress response, apoptosis, and survival. Both pathways are also involved in the development of cancer.
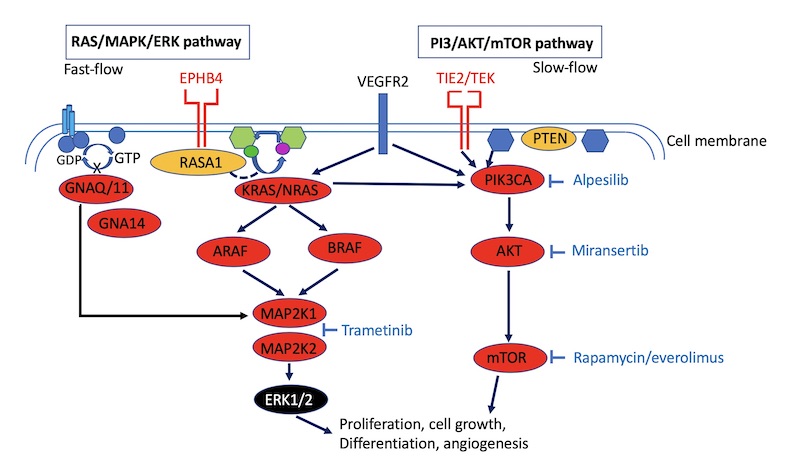
Figure 1: The RAS/MAPK/ERK-pathway and the PI3/AKT/mTOR pathway
TIE2
Sixty percent of vascular malformations (VMs) have an activating mutation (gain-of-function) in the TIE2 protein. TIE2 is part of the mTOR pathway. When the mTOR pathway is triggered by TIE2, endothelial cell growth occurs. Such an activating mutation causes the effect of TIE2 to become stronger or even abnormal. In this case this can lead to abnormal growth of endothelial cells. Twenty percent of VMs have an activating mutation in the PIK3CA gene in the mTOR pathway.
ISSVA-classification
The ISSVA classification (https://www.issva.org/classification) describes the various types of malformations but also the mutation(s) associated with a specific malformation is indicated (as far as known). So for example the subspecies cutaneomucosal VM (VMCM), common VM, multifocal VM and blue rubber bleb nevus (BRBN) are all associated with TIE2 mutations.
PIK3CA-gen
The most common mutations in the PIK3CA gene are associated with cancer and lymphatic malformations and overgrowth syndromes, such as Klippel-Trenaunay syndrome, congenital lipomatous overgrowth, vascular malformation, epidermal nevi, scoliosis/skeletal and spinal syndrome (CLOVES), and megalencephaly-capillary malformation.
Studies with sirolimus among patients with vascular malformations
Sirolimus is a direct inhibitor of mTOR, blocking signaling (figure 1) and protein synthesis, resulting in antitumoral and antiangiogenic effects. The first report of significant clinical response of sirolimus in vascular malformations was the description of six patients with complex life-threatening vascular anomalies in whom sirolimus was given in a compassionate use setting [2]. It concerned patients with KHE (kaposiform hemangioendothelioma) and Kasabach-Merritt phenomenon (KMP) with severe coagulopathy (uncontrolled bleeding) and high-output heart failure that resolved after treatment with sirolimus. One patient with capillary-lymphatic VM, and four patients with diffuse microcystic LMs with an abnormal increase in the amount of fluid in the chest cavity requiring chest tubes that could be removed after treatment with sirolimus.
The largest (Phase II) study to date, was conducted in 2016 [3] on 61 patients with complex vascular anomalies (with complications such as uncontrolled bleeding, chronic pain, recurrent cellulite, ulcers, intestinal and bone problems, heart failure). 57 were suitable for further evaluation. 83 per cent had a partial response after 6 months, 85 per cent had a partial response after 12 months. In general, these patients had a significant improvement in quality of life.
A Phase III study (VASE) in which sirolimus is evaluated in children and adult patients with complex vascular malformations (of the veins) who respond poorly to standard treatment (EudraCT Number: 2015-001703-32) is now underway. Patients receive sirolimus for 2 years but may be retreated after the end of treatment in the case of relapse of symptoms.
Sirolimus and other inhibitors
The mTOR inhibitor sirolimus is the most extensively studied drug to date in this context. Promising results from various Phase I and II prospective studies and from retrospective research have led to a Phase III clinical trial (VASE) currently being conducted in Europe. Evidence for the successful use of other targeted compounds in other indications, such as the PIK3CA inhibitor alpelisib, the MEK inhibitor trametinib and the monoclonal anti-VEGF antibody bevaci-zumab, is also increasing, but needs further careful study.
Side effects
Since sirolimus and other inhibitors interfere with important processes such as cell division and growth, cell cycle regulation, proliferation, protein synthesis and cell survival, there are also side effects. The most common side effects are: headache, fatigue, rash, inflammation of the oral mucosa, nausea and diarrhoea. The dose is very important. The mTOR pathway is also a central regulator of the immune system. If that is suppressed, it is very important to keep an eye on side effects such as infections.
References
Composed by Lilian Vermeer
[1] Van Damme A,· Seront E, Dekeuleneer V,· Boon LM, Vikkula M. New and Emerging Targeted Therapies for Vascular Malformations, American Journal of Clinical Dermatology, 15 juni 2020
https://doi.org/10.1007/s40257-020-00528-w
[2] Hammill AM, Wentzel MS, Gupta A, Nelson S, Lucky A, Elluru R, et al. Sirolimus for the treatment of complicated vascular anomalies in children. Pediatr Blood Cancer. 2011;57:1018–24.
[3] Adams DM, Trenor CC 3rd, Hammill AM, Vinks AA, Patel MN, Chaudry G, etal. Efficacy and safety of sirolimus in the treatment of complicated vascular anomalies. Pediatrics. 2016;137(2):e20153257.
