
The article discusses three areas wearable device manufacturers and service providers can focus to ensure success in the market:
1. Meeting and exceeding user needs
2. Delivering rigorous, compliant data and insights for the entire patient care ecosystem
3. Crafting a viable business model
While wearable medical devices1 offer the opportunity to monitor, diagnose, and deliver individualized care to patients, adoption by clinicians has yet to gain significant traction. However, the COVID-19 pandemic has raised awareness of wearables and how they can provide clinical benefit in a remote setting. Many medical practitioners and patients were introduced to new ways of delivering care, including expansion of existing telemedicine and remote patient monitoring.
Traditionally, mainstream wearables familiar to consumers were not developed with clinical rigor from the outset. Medical devices have a high bar for accuracy and validation to integrate and deliver the type of data required for clinical use within the healthcare delivery ecosystem. Consumer devices often leverage less-accurate sensors or inferred measurements to support informational and non-medical feedback to the user. Seeing the evolving remote care trends and revenue potential of clinical use, consumer device makers have begun closing that gap. For example, Apple has received FDA clearance for the ECG function on the Apple Watch2, opening the door for its use in clinical settings. Oura, maker of a smart ring that senses activity, sleep, body temperature, and heart rate variability, has partnered with UCSF3 to create an in vivo trial to rapidly collect correlative data between biomarker changes and COVID-19 diagnoses to inform a diagnostic algorithm.
In fact, research shows the wearables market is estimated to grow considerably, with 19 percent CAGR from 2020 to 2030, representing potential economic value of $0.5-$1.8 trillion by 2030.4 This growth includes consumer, industrial, and medical applications, but market activity indicates significant promise (and movement) in the healthcare domain:
- In 2020, global shipments of hearables, watches, wristbands, and other wearables stands at 444.7 million units5
- Investors have committed more than $400 million to wearables-focused startups since 20156
- Large medical device companies are acquiring smart devices, such as Stryker’s acquisition of OrthoSensor7 and Boston Scientific’s acquisition of Preventice Solutions8
- Academia continues to drive innovation in ever smaller technology, with advances in implantable chips from UC Berkeley,9 flexible batteries from Stanford University,10 and flexible sensors from the University of Illinois at Urbana-Champaign11
Device manufacturers are well positioned to capitalize on this unique opportunity. However, to deliver devices useful for clinicians and patients, wearable technologies must advance further. Breakthroughs in healthcare will likely come from devices that not only accurately measure disease-specific biomarkers at home but also provide real-time contextual data on other features that affect disease management. These insights must be shared via a simple user experience to encourage patient adoption and must demonstrate improved medical care and reduced costs to providers and payers.
Based on our experience, wearable device manufacturers and service providers should focus on three areas to ensure success in the market:
- Meeting and exceeding user needs
- Delivering rigorous, compliant data and insights for the entire patient care ecosystem
- Crafting a viable business model
Here, we provide an overview of each focus area. In future articles, we’ll dive more deeply into each.
1. Meeting And Exceeding User Needs
Patients and clinicians expect sufficient long-term benefits in exchange for using wearable devices, what can be called “return on engagement.”12
For patients, return on engagement translates into benefits such as reassurance that a professional is monitoring them and able to deliver care above and beyond what point-in-time visits can offer. They expect this to come with minimal discomfort from the ongoing use of these devices. If the device is uncomfortable or creates substantial burden on patients’ daily routines, they won’t use it. As a result, strive to develop smaller, less obtrusive devices with longer battery life. Device designers must also understand how physical and cognitive ergonomics affect device operation and data comprehension. Patient interactions with the device should be simple, seamless, engaging, and personal.
Clinicians’ return on engagement can be realized by integrating device read-outs into their current systems and workflows seamlessly, as well as by generating insights that they wouldn’t be able to infer otherwise. Devices and associated services that combine wearable data with real-world data sources to reveal patterns and trends will give clinicians a more comprehensive picture of patient health compared with point measurements in the doctor’s office, enabling more efficient disease management.
For both clinician and patient, safety, data security, and efficacy are table stakes, with correlation to better patient outcomes the ultimate sign of success.
2. Delivering Rigorous, Compliant Data And Insights For The Entire Patient Care Ecosystem
The data collected by wearables must be sensitive, specific, clinically validated, and offer more than that available through current standards of care. Such rich data will enable a better understanding of disease progression, more precise patient segmentation, and, ideally, better clinical outcomes.
However, the data alone won’t provide this level of value. Wearables must also deliver a level of contextualization and analysis that requires robust algorithm development hand-in-hand with device development (e.g., to accommodate individual variations and provide clear clinical insights). These insights must then be visualized and displayed in a way that is easy for users to comprehend and act upon.
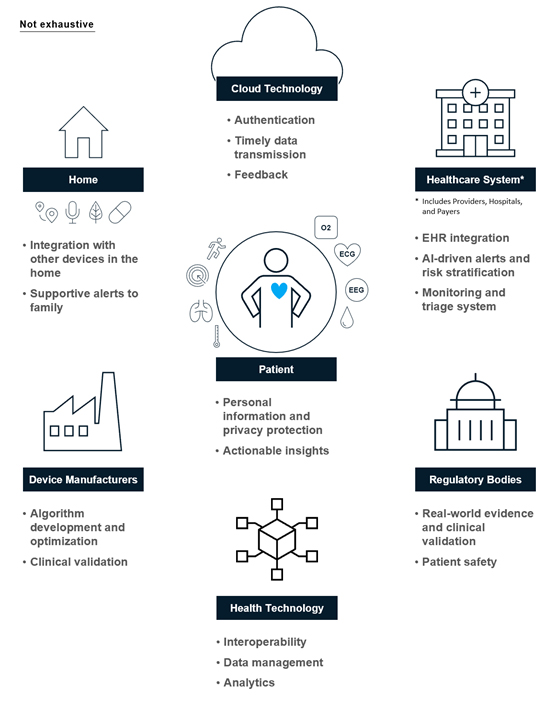
Device makers must also consider the needs and requirements of other stakeholders in the patient care ecosystem, including payers, hospitals, and regulators. Data serves as the connective tissue that enables this ecosystem to better work together for the good of the patient, making it critical that these stakeholders can share and communicate the data and insights seamlessly. Industry standard protocols such as FHIR or HL713 enable interoperability to ensure all stakeholders have access to the right information at the right time. As a result, consider how your device’s data will be transferred to the electronic health record for clinician review or how it will interact with third-party APIs.
Finally, data storage and transfer must comply with industry regulations — such as HIPAA, GDPR, PIPEDA, and CCPA14 — and best practices for privacy and security. For instance, use industry-standard encryption for data on devices and during transmission, and prevent or mitigate unauthorized access to devices, smartphones, and servers. As regulations evolve, such as with the European Commission’s recently announced legal framework for trustworthy AI, so must manufacturer approaches.
Figure 1: Device makers should consider the ecosystem of stakeholders vested in patient health as they develop their offerings.
3. Crafting A Viable Business Model
With the wearables market expected to become increasingly attractive and competitive, wearable device manufacturers and service providers will need to not only get the user experience and data right but also ensure their value creation and capture model helps them stand out from the crowd. The details matter, and the choices made in the earliest stages can become differentiators, from strategic decisions to logistics and delivery models.
To start, you must define your go-to-market plan by answering key questions:
- Who is the primary target customer — hospital systems, patients, payers, or some combination of these?
- Are devices reimbursable or an out-of-pocket expense?
- What technology, integrations, or partnerships may be required?
The answers to these questions affect both the design of the product and the business as you seek to demonstrate value over the current quality and cost of care. The calculation may seem simple at first glance, but it involves detailed mapping of and interviews with the stakeholders in the ecosystem, in-depth analysis of claims data and outcomes, and rapid iteration in response to feedback. Only then may you have the information needed to define the value proposition and set a price point.
As mentioned previously, existing devices already capture a lot of data, but clinical applications need more detailed, nuanced information and must meet the standards for clinical validation. Medical devices, including combination products like drug delivery devices, also must meet quality and regulatory requirements (though these may differ for FDA Class I through Class III devices) to ensure their success. Therefore, new market entrants should consider whether to buy or build capabilities enabling them to meet these thresholds based on a clear understanding of the company’s risk profile and an honest assessment of its current capabilities.
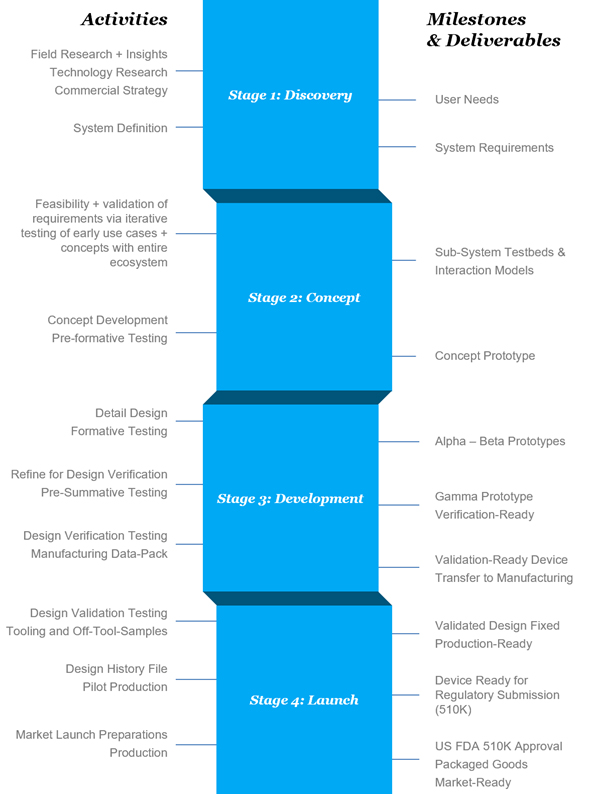
As shown in Figure 2, bringing a new electromechanical medical product to market is complex. However, with a great team and smart processes, companies can successfully move a product through the phases from early research and discovery to launch.
Figure 2: The product development process for a medical device has four stages.
Conclusion
Wearables have enormous potential to change the way healthcare is defined and delivered, transferring care beyond the four walls of the hospital to ambulatory and home settings. Wearables can close the gaps in accessible healthcare by taking advantage of the changes accelerated by COVID-19, rapidly improving technology, and user-driven insights to enable better disease understanding, diagnoses, and care.
As you innovate in this space, you must navigate the complex interplay of creating products that are delightful to use, deliver clinically validated and actionable data, and create value within a solid, scalable business model. Companies that can address these three areas well will be primed for success.
About The Authors:

Sara Cinnamon is an expert with McKinsey Design focusing on all aspects of the product development lifecycle, including strategy, design, quality, and manufacturing. Sara also leads the wearable pillar of the HealthTech Network. As an expert practitioner, she combines her background in systems engineering and agile methodology to successfully address complex product and user challenges. She is based in San Francisco.

Amy Hung is a director of client capabilities in the McKinsey New Jersey office, where she leads the Global Life Sciences Intelligence Team and co-leads the Global HealthTech Network. With more than 15 years of experience in the life sciences, she has worked with a variety of healthcare companies on growth strategy, capability development, digital and analytics transformations, and org and operating model design. She leads the Annual McKinsey Digital Health Conference, has co-authored a number of papers on digital health and digital in pharma, and spends a good portion of her time speaking with and collaborating with innovators in healthtech. She has a B.S. in chemical engineering from MIT and a dual M.S. in materials science engineering and medical engineering and medical physics from the MIT-Harvard Health Sciences and Technology (HST) Program.

Tobias Silberzahn is a trained biochemist and immunologist and works as a partner in McKinsey’s Berlin office, where he is a member of the Lifescience Practice. Over the last 12 years with McKinsey, Tobias has served mainly health tech, pharmaceutical, medical device companies, and ministries of health. He leads the global HealthTech Network, a community of more than 1,000 health tech companies, and he hosts the quarterly HealthTech CEO Roundtable. As part of his work, Tobias also hosts the MedTech R&D Industry Roundtable. Within McKinsey, Tobias also leads the Health & Wellbeing program, focused on sleep, nutrition, stress management and fitness.

Eli Weinberg is a partner in McKinsey’s New York office, leader in our Life Science R&D practice, and co-leader of our HealthTech Network in North America. Eli advises organizations of all types and all sizes that are innovating in life science and health technology. Prior to McKinsey, he was directly involved in health tech in both academia and venture-backed startups. Eli holds a B.S., M.S., and Ph.D. from the Massachusetts Institute of Technology and was a postdoctoral fellow at UC Berkeley.
- The devices considered here include wearables (such as wrist-worn actigraphs, devices adhered to the body like continuous glucose meters, stickers that sense biomarkers in sweat, or clothing that measure joint range of motion to support physical therapy), nearables (devices positioned in the room, such as ballistic respiratory monitors, or handhelds, such as smart inhalers), ingestibles (such as swallowable smart pills or endoscopic cameras), and implantables (such as neurostimulators or smart implants).
- https://www.apple.com/healthcare/docs/site/Apple_ECG_app_during_COVID-19.pdf
- https://ouraring.com/blog/ucsf-tempredict-study/
- https://www.mckinsey.com/business-functions/mckinsey-digital/our-insights/iot-value-set-to-accelerate-through-2030-where-and-how-to-capture-it
- https://www.statista.com/statistics/437871/wearables-worldwide-shipments/
- https://www.crunchbase.com/search/funding_rounds/2e065220766d1c34fb9584da83cd3960
- https://www.massdevice.com/stryker-acquires-orthosensor-and-its-knee-surgery-sensor-tech/
- https://news.bostonscientific.com/2021-01-21-Boston-Scientific-Announces-Agreement-To-Acquire-Preventice-Solutions-Inc
- https://news.berkeley.edu/2021/04/14/tiny-wireless-implant-detects-oxygen-deep-within-the-body/
- https://engineering.stanford.edu/magazine/article/new-stretchable-battery-can-power-wearable-electronics
- https://www.techtimes.com/articles/102165/20151102/thin-flexible-sensor-tracks-blood-flow-under-skin.htm
- “Return on engagement” coined by Bettina Ryll from Melanoma Patient Network Europe
- FHIR = Fast Health Interoperability Resources; HL7 = Health Level Seven
- HIPAA = Health Insurance Portability and Accountability Act of 1996; GDPR = General Data Protection Regulation; PIPEDA = Personal Information Protection and Electronic Documents Act; CCPA = California Consumer Privacy Act
Discover more about McKinsey:
McKinsey is a global management consulting firm committed to helping organizations realize sustainable, inclusive growth. The Firm works with clients across the private, public, and social sectors to solve complex problems and create positive change for all their stakeholders. McKinsey combines bold strategies and transformative technologies to help organizations innovate more sustainably, achieve lasting gains in performance, and build workforces that will thrive for this generation and the next.
