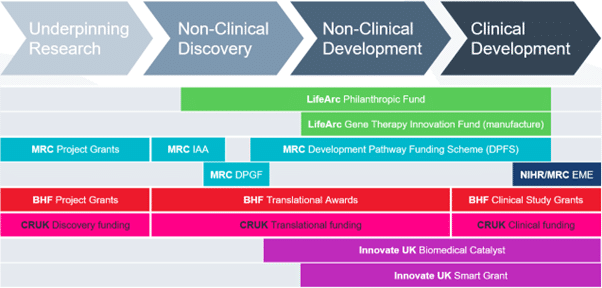
Overview
The Gene Therapy Innovation Fund (GTIF) aims to support the advance of promising gene therapies for people living with rare conditions. There will be no expectation of a return to LifeArc on this funding, but projects must have a credible technical and financial path to significant patient benefit if they are successful. The GTIF is a £5M annual fund. The funding panel reviews full applications and makes funding decisions twice a year.
The fund provides grants to academic researchers working to advance new gene therapies and who require viral vector material manufactured to GMP (or GMP-like) grade in order to support the programme. Funding is awarded for academics to use the manufacturing capabilities of the Innovation Hubs for Gene Therapies, and closely associated work. This is likely to include manufacturing process development, process scale-up, development of analytical methods, production of technical batches and batches manufactured in accordance with Good Manufacturing Practice (“GMP”) guidelines, to support regulatory studies and/or clinical trials. Where projects include substantial non-clinical and/or clinical studies, it is expected that applicants will secure co-funding from a suitable scheme, such as LifeArc’s Philanthropic Fund, to cover the wider translational aspects of their project, e.g., GLP safety/toxicity and/or clinical trial costs. Where the manufacturing aspects constitute part of a larger project, funding under GTIF may be conditional upon securing suitable co-funding.